The control of cleanliness during the electroslag remelting (ESR) process of die steel 1.2083 is as follows:
Die steel is an important industrial material widely used in aerospace, automotive manufacturing, electronics, toys, medical devices, and many other fields. To improve the cleanliness, wear resistance, and impact resistance of this steel to meet user requirements, research was conducted on the control of the ESR process for this series of steels. This paper focuses on the causes and metallurgical mechanisms of excessive Class C inclusions during the steelmaking and ESR processes, studying the composition of the pre-melted slag for ESR and the ESR process technology (deoxidant usage). By optimizing process parameters, the issue of excessive Class C inclusions was reduced, producing products that meet user requirements.
Industrial trial for 1.2083 steel:
1.1 Industrial trial process
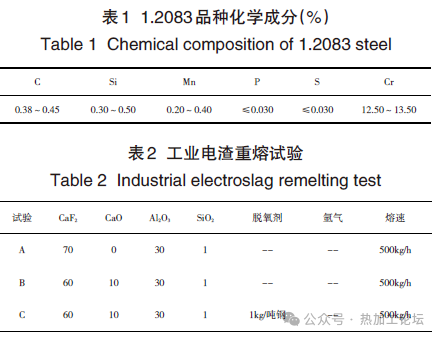
The production process flow for 1.2083 steel is as follows: initial smelting furnace (100-ton converter, 100-ton electric furnace, 50-ton alloy melting furnace) → 50-ton AOD refining furnace → 50-ton LF refining furnace → continuous casting of 180 square billets → ESR of 5-ton ingots → slow cooling annealing of ingots → heating and rolling of ingots → slow cooling annealing of rolled products → storage. The rolled product dimensions are 30-120 mm thick × 300-610 mm wide. The ESR involves welding 180 square continuous cast billets into a “田” shape billet, using a crucible with a diameter of Φ660 mm. Three industrial ESR trials were carried out using a constant-power 5-ton non-atmosphere-protected double-arm ESR furnace, with various process parameters listed in Table 2.
1.2 Melting composition, inclusions, and slag system components of the steel.
Comparison of test data before and after different ESR process trials:
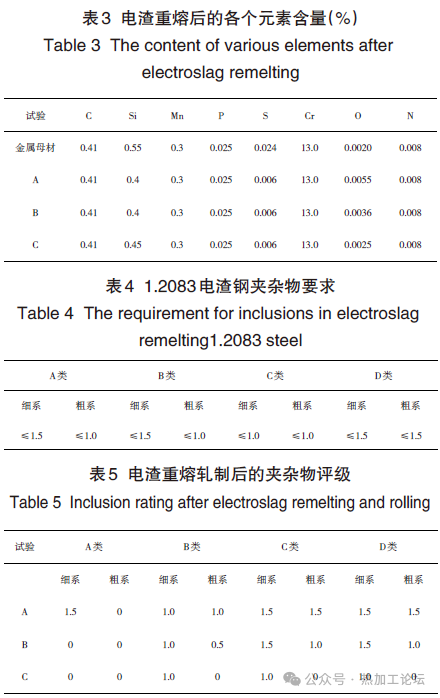
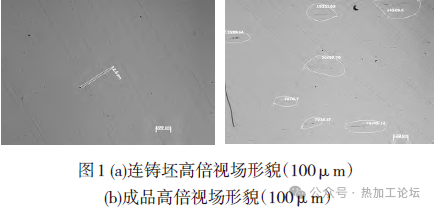
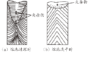
The composition of the ingots before and after ESR is shown in Table 3, with corresponding inclusion ratings given in Table 5. Using the original Process A, more than 30 ESR ingots produced simultaneously showed that the finished rolled products contained Class C fine or coarse inclusions exceeding the standard by 0.5 to 1.5 grades, reaching levels of 1.5 to 2.5 grades according to high-magnification inspection (inclusion detection according to GB/T10561-2023-A method). The inclusions in the original billets (continuous cast billets) were quite clean, with inclusion control levels higher than those of the ESR finished products using Process A. No batch occurrences of Class C (silicate inclusions) were found in the metallic billets. Analysis of four original billets revealed [O] content controlled at ≤20×10^-6. Testing several ESR finished products using Process A showed [O] content controlled at 50-60×10^-6, indicating significant oxygen increase during the ESR process, influencing inclusion generation. See Fig. 1(a) and (b).
Using Process B, the oxygen content was lower than with Process A, and the inclusion control level was higher than with Process A. Using Process C further reduced the oxygen content in the ESR ingots, bringing the inclusion ratings of the rolled products within the required standards.
Analysis of the results of different ESR process trials:
Metallurgical analysis of the trial results showed that the original electrode billets were relatively clean, with oxygen content at 20×10^-6. After adopting Process A, the oxygen content increased to above 50×10^-6. After increasing the CaO content in the slag with Process B, the oxygen content was reduced to 36×10^-6. With Process C, increasing the CaO content in the slag and adding deoxidant at a rate of 1 kg/ton of steel brought the oxygen content down to 25×10^-6.
With Process A using the three-seventy pre-melted slag, containing approximately 1% SiO2 impurities, ordinary ESR furnaces contain large amounts of oxygen. At high temperatures, the metallic electrode undergoes surface oxidation (4Fe+3O2=2Fe2O3), causing large amounts of Fe2O3 to enter the slag, raising the oxygen potential of the slag, i.e., increasing FeO content sharply. According to the reaction [Si]+2(FeO)=SiO2+2[Fe], a significant amount of SiO2 is generated in the steel, some of which remains in the ESR ingot, leading to excessive Class C inclusions in the rolled products. Some of the SiO2 enters the slag, significantly increasing the activity of SiO2 in the slag. According to the reaction [Si]+2[O]=(SiO2), when the SiO2 content in the slag increases significantly, the corresponding equilibrium oxygen in the steel also rises, thus the oxygen content in the ESR ingot rises to 55×10^-6.
Using Process B with increased CaO in the slag, the CaO can combine with SiO2 in the slag to form CaO·SiO2, reducing the activity of SiO2. According to the reaction [Si]+2[O]=(SiO2), when the SiO2 content in the slag rises, part of the SiO2 is combined into CaO·SiO2, which can reduce the activity of SiO2, thus the equilibrium oxygen in the steel is lower than with Process A’s three-seventy pre-melted slag, at 36×10^-6, correspondingly improving the inclusion levels.
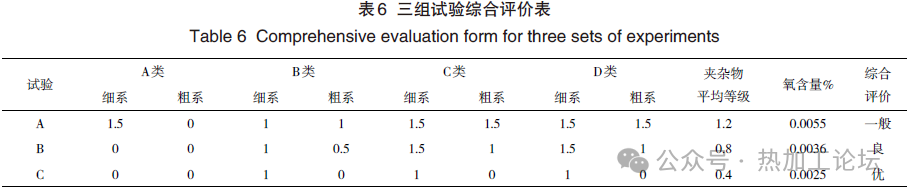
Using Process C with 1 kg/ton of steel deoxidant aluminum powder, the aluminum powder reacts with FeO in the steel to form Al2O3+3Fe, reducing the content of unstable FeO and suppressing the reaction [Si]+2(FeO)=SiO2+2[Fe]. The activity of SiO2 in the slag increases very slowly. According to the reaction [Si]+2[O]=(SiO2), when the SiO2 content in the slag changes little, the corresponding equilibrium oxygen in the steel is also low, thus the oxygen content in the ESR ingot is further controlled to 25×10^-6, correspondingly reducing the inclusion levels to meet product standards. Comprehensive evaluation is provided in Table 6 and Fig. 2.
Improvement measures for the ESR process:
With the continuous development of high-quality special steels through ESR, the requirements for products are becoming increasingly stringent. Preventing the oxidation of metallic electrodes and reducing the oxygen content in ESR ingots will become key trends in the future. Adopting atmosphere-protected ESR technology to prevent the oxidation of metallic electrodes is an important method to enhance ESR products. Therefore, our company has introduced atmosphere-protected ESR modification technology from Soochow University, capable of reducing the oxygen partial pressure in the ESR furnace to 1×10^-6 levels, comparable to that of atmosphere-protected ESR furnaces, as shown in Fig. 3, enhancing the competitiveness of our products overall.
In summary, raising the alkalinity of pre-melted slag, adding deoxidants, and adopting atmosphere-protected ESR modification technology are effective means of reducing inclusions. Through the research and optimization conducted here, we smelted and rolled five ESR ingots, sampling the head and tail of the finished products to inspect the Class C fine and coarse inclusions, all rated at Level 0, meeting the process requirements.
Conclusions:
(1) Excessive Class C inclusions in 1.2083 ESR steel are due to the surface oxidation of metallic electrodes during remelting, with the oxide scale entering the slag, increasing the oxygen potential in the slag. Silicon in the molten steel is continuously oxidized by FeO in the slag, increasing the SiO2 content and activity in the slag, leading to increased equilibrium oxygen and higher inclusion content in the ESR ingot.
(2) Process research and optimization of slag system ratios, increasing CaO to 10%, and adding strong deoxidants during smelting, with 1 kg of deoxidant added every hour during remelting, can effectively suppress the SiO2 content in the slag and reduce the grade of Class C inclusions.
(3) Through analysis of the causes affecting cleanliness and process optimization, the products produced meet user requirements.